Anaplastic lymphoma kinase (ALK) is one of the important driving factors in Non-small cell lung cancer (NSCLC) patients. The ALK fusion mutation is presented at approximately 5% in NSCLC patients and is usually common in young, non-smokers or mildly smoked lung adenocarcinoma patients. Activation of ALK activates downstream signaling pathways, leading to tumorigenesis and survival. ALK inhibitors can effectively inhibit the activity of ALK, thereby inhibiting tumor growth.
Ensartinib hydrochloride has a strong affinity to ALK. The China phase II registration study has recruited 160 patients and aims to evaluate the efficacy, safety and biomarkers of ensartinib for ALK+ NSCLS patients who are resistant to crizotinib. By mid-September, 2018, the results assessed by the independent review committee (IRC) indicated the 52% ORR, 93% DCR, intracranial ORR and DCR as 70% and 98%, respectively, reaching the primary endpoint of the study. The most common AE was moderate rash, and it could be recovered or relieved with 10% dose reduction or other targeted treatments, showing a good and controllable safety profile. The clinical results revealed that ensartinib hydrochloride has a better efficacy profile than other ALK inhibitors, especially in patients with intracranial metastasis. | 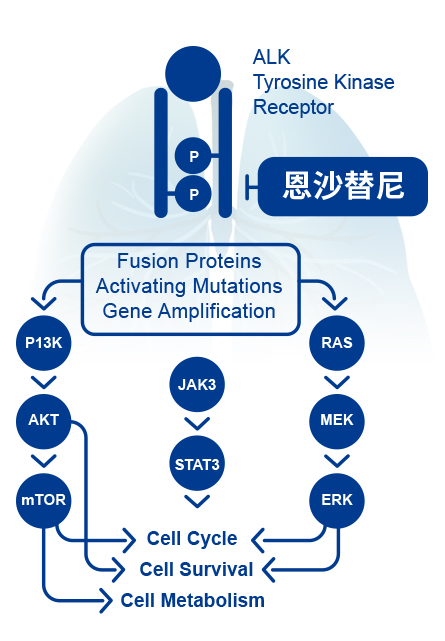
|

